- Key Types of Equipment Supplied in Pharma Manufacturing
- Engineering Standards and Compliance in Pharma Equipment
- Selecting a Pharmaceutical Equipment Supplier—Critical Factors
- Latest Technology Trends Shaping Pharma Equipment
- Ensuring System Reliability and Quality: SKE & Eagle’s Approach
- Practical Applications and Case Studies
- Frequently Asked Questions
- Contact and Next Steps
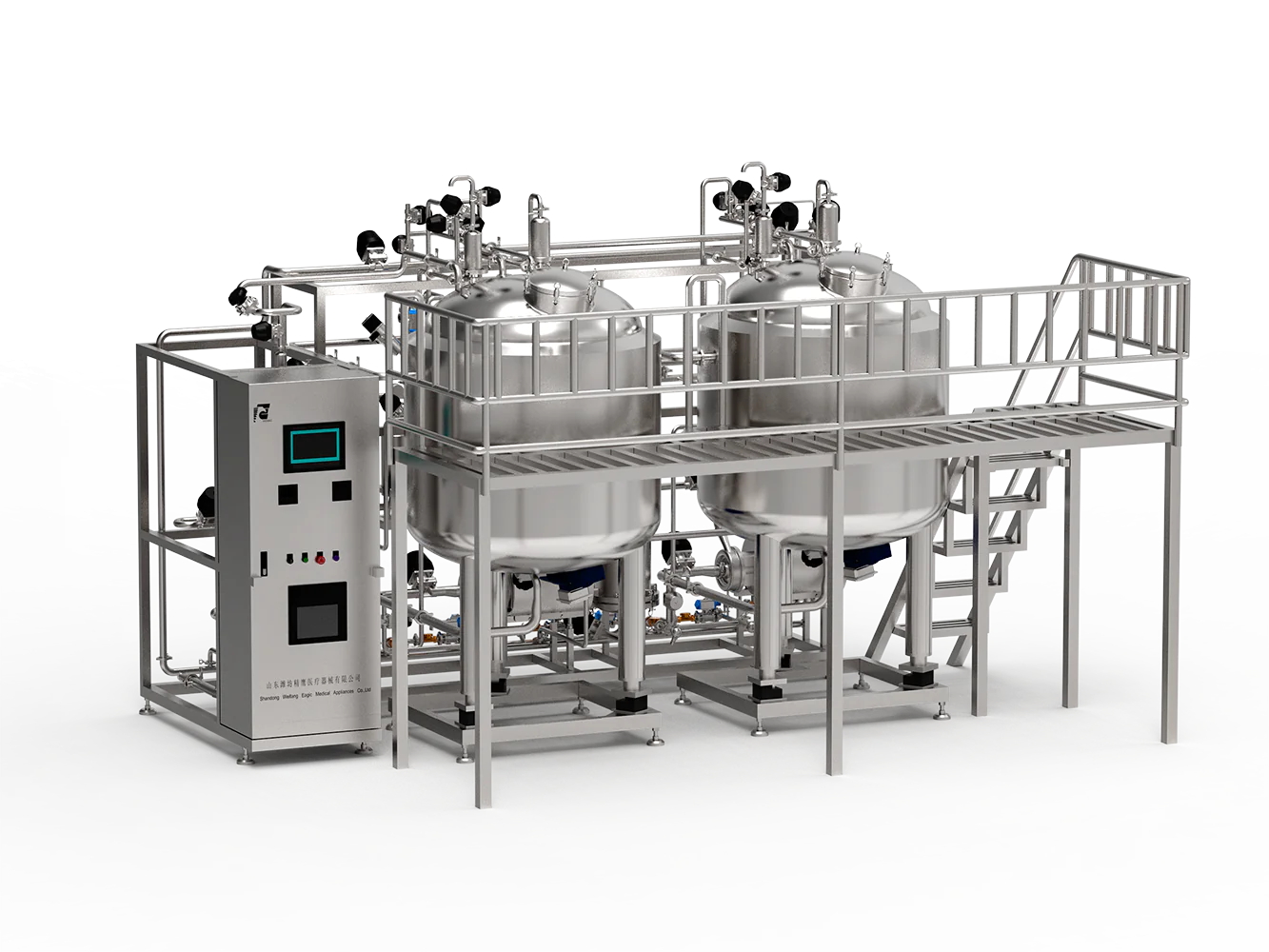
Key Types of Equipment Supplied in Pharma Manufacturing
Pharmaceutical equipment encompasses a broad range of machinery vital for drug production, processing, packaging, and quality control. A proficient pharmaceutical equipment supplier must cater to this extensive spectrum, which typically includes:
- Water Treatment Systems: Production-grade water is essential in pharmaceutical formulations. SKE & Eagle’s water treatment solutions—such as reverse osmosis units and advanced filtration systems—ensure compliance with USP Purified Water and Water for Injection specifications.
- Sterilizers and Autoclaves: Equipment designed to eradicate microbial contamination through steam sterilization, vital for aseptic manufacturing and medical device production.
- Mixing and Blending Machines: Critical for preparing homogeneous pharmaceutical solutions, suspensions, and emulsions while maintaining material integrity.
- Filling and Packaging Equipment: Precision machinery for sterile filling of vials, ampoules, and syringes, including inspection systems that guarantee dosage accuracy and container integrity.
- Analytical Instruments: Inline and at-line devices supplied ensure real-time monitoring of product quality parameters and process variables.
Beyond individual equipment, pharmaceutical equipment suppliers increasingly provide integrated systems—combining multiple processes into automated workflows, enhancing throughput, reducing operator intervention, and facilitating compliance with process analytical technology (PAT) frameworks.
Engineering Standards and Compliance in Pharma Equipment
Proper adherence to engineering standards is non-negotiable in pharmaceutical manufacturing because substandard equipment can compromise product safety and efficacy. A responsible pharmaceutical equipment supplier must design and fabricate according to exacting standards, including but not limited to:
- Good Manufacturing Practices (GMP): Guidelines ensuring that equipment design facilitates cleanliness, sterilization, and traceability.
- ISO 13485: Focuses on design and manufacturing processes for medical device equipment, which overlaps with pharma bioprocess machinery.
- ASME BPE: The American Society of Mechanical Engineers’ BioProcessing Equipment standards set forth requirements for materials, surface finishes, and design criteria specifically for bio- and pharmaceutical manufacturing.
- FDA Regulations: Includes 21 CFR Part 11 regarding electronic recordkeeping and 21 CFR Part 820 for quality system regulations.
SKE & Eagle exemplifies compliance, utilizing validated design processes and rigorous quality management systems that meet or exceed these standards. For instance, SKE & Eagle integrates advanced CAD modeling, finite element analysis (FEA) for stress and fatigue testing, and surface roughness validation to ensure equipment robustness and operational longevity.
Additionally, compliance demands comprehensive documentation packages from the supplier, encompassing IQ/OQ/PQ (Installation, Operational, and Performance Qualification) protocols, traceability matrices for raw materials, and maintenance guidelines. This documentation underpins manufacturers’ validation activities and regulatory submissions.
The pharmaceutical equipment supplier’s engineering must also anticipate operational conditions—such as CIP (Clean-In-Place) and SIP (Steam-In-Place) cycles—to ensure the equipment’s resilience during aggressive cleaning without degradation, thus safeguarding product purity.
Selecting a Pharmaceutical Equipment Supplier—Critical Factors
Choosing the right pharmaceutical equipment supplier is a multifaceted decision that directly influences manufacturing efficiency, product quality, and compliance. Key considerations include:
- Industry Experience and Expertise: Suppliers should demonstrate a robust track record with pharmaceutical clients and a deep understanding of pharma-specific demands. SKE & Eagle’s long-standing experience with water treatment systems and precision-engineered solutions exemplifies this prerequisite.
- Customization Capability: A successful supplier can tailor equipment to specific formulations, batch sizes, and process requirements, accommodating scale-up and diverse modalities such as sterile injectables or oral solids.
- Regulatory Support: Equipment must be designed and accompanied by documents facilitating easy validation and FDA or EMA audits.
- Technological Innovation: An advanced supplier incorporates modern control systems such as PLCs, SCADA integration, and Industry 4.0 data analytics compatibility, offering manufacturers data-driven process optimization tools.
- After-Sales Support and Service Network: Reliable technical support, spare parts availability, and service agreements minimize downtime risk critical to continuous pharmaceutical operations.
- Quality Assurance and Certifications: Assess supplier adherence to ISO quality standards and documented quality processes guaranteeing consistency and reliability.
Engaging suppliers like SKE & Eagle, which emphasize systemic engineering and lifecycle support, allows manufacturers to focus on core drug development and commercialization while trusting equipment integrity and performance.
Latest Technology Trends Shaping Pharma Equipment
The pharmaceutical equipment supplier landscape is evolving rapidly with technological advances improving productivity, compliance, and sustainability:
- Automation and Smart Systems: Automated process controls, augmented with AI-driven predictive maintenance, reduce human error and unplanned downtime.
- Modular Equipment Design: Enables flexible manufacturing lines adaptable to multiple products and fast changeovers, a necessity in personalized medicine production.
- Non-Contact and Single-Use Technologies: The rise of disposable bioprocess bags and single-use filling systems minimizes cleaning requirements and cross-contamination risks.
- Advanced Materials and Surface Coatings: New alloys and antimicrobial coatings extend equipment lifespan and further enhance contamination control.
- Real-Time Process Monitoring: Integration of sensors and analytical tools facilitates continuous quality verification inline, aligning with Process Analytical Technology (PAT) initiatives.
At SKE & Eagle, these trends are reflected in their solutions portfolio, where advanced water treatment plants feature IoT-enabled monitoring, and equipment modules support rapid redeployment. Such innovation underscores not just equipment supply but delivering future-ready systems designed for evolving pharma requirements.
Ensuring System Reliability and Quality: SKE & Eagle‘s Approach
Reliability is a critical dimension of pharmaceutical equipment performance. Failures in equipment can lead to costly batch rejections, regulatory inspections, and endanger patient safety. SKE & Eagle’s engineering philosophy centers on robust design, precise manufacturing, and a disciplined quality system.
This begins with stringent material selection, often favoring corrosion-resistant stainless steel and validated water treatment membranes conforming to USP standards. Their manufacturing processes incorporate high-precision machining and surface finishing techniques to meet smoothness and cleanliness benchmarks essential for bio-containment.
Additionally, SKE & Eagle employs redundant instrumentation and fail-safe control architectures in their systems, providing multiple layers of fault detection and operational safeguards. The company’s comprehensive testing protocols incorporate pressure, flow, temperature, and contamination control verifications prior to delivery.
Their commitment to quality is further demonstrated through extensive documentation, training, and on-site service capabilities. This holistic approach ensures pharmaceuticals manufacturers can achieve sustained compliance, minimize operational risks, and maintain continuous production integrity.
Frequently Asked Questions
What qualifications should I look for in a pharmaceutical equipment supplier?
Look for suppliers with proven pharmaceutical industry experience, adherence to GMP and ASME BPE standards, a comprehensive quality management system, strong regulatory documentation support, and a reputation for customization and service excellence.
How does a pharmaceutical equipment supplier help ensure regulatory compliance?
Suppliers design and fabricate equipment to meet industry standards such as GMP and FDA regulations, provide detailed validation documentation (IQ, OQ, PQ), and support installation and qualification processes, thereby facilitating smooth regulatory inspections and audits.
What are the advantages of choosing a supplier like SKE & Eagle?
SKE & Eagle offers advanced water treatment systems, precise manufacturing capabilities, and integrated solutions designed to meet pharmaceutical challenges. Their engineering standards, focus on reliability, and comprehensive lifecycle support deliver enhanced operational efficiency and compliance confidence.
What types of pharmaceutical equipment are most commonly supplied?
Common equipment includes water purification systems, sterilizers, filling and packaging machines, mixers, and analytical instruments, all tailored for pharmaceutical manufacturing environments with strict hygiene and control requirements.
Can pharmaceutical equipment be customized for specialized processes?
Yes, leading suppliers provide customization to accommodate specific formulations, batch sizes, or technology platforms. This ensures equipment aligns with unique manufacturing processes and regulatory needs.
Connect with SKE & Eagle for Pharmaceutical Equipment Excellence
Interested in learning how a trusted pharmaceutical equipment supplier can elevate your manufacturing capabilities? SKE & Eagle combines engineering expertise with industry-leading water treatment and processing solutions to support your operational and compliance goals.
Reach out via info@ske-eagle.com or visit our Facebook page to explore the latest innovations. We invite you to fill out our contact form at the bottom of the website for personalized consultation and support.
Partner with SKE & Eagle for equipment solutions that deliver uncompromising quality, reliability, and performance in pharmaceutical manufacturing.