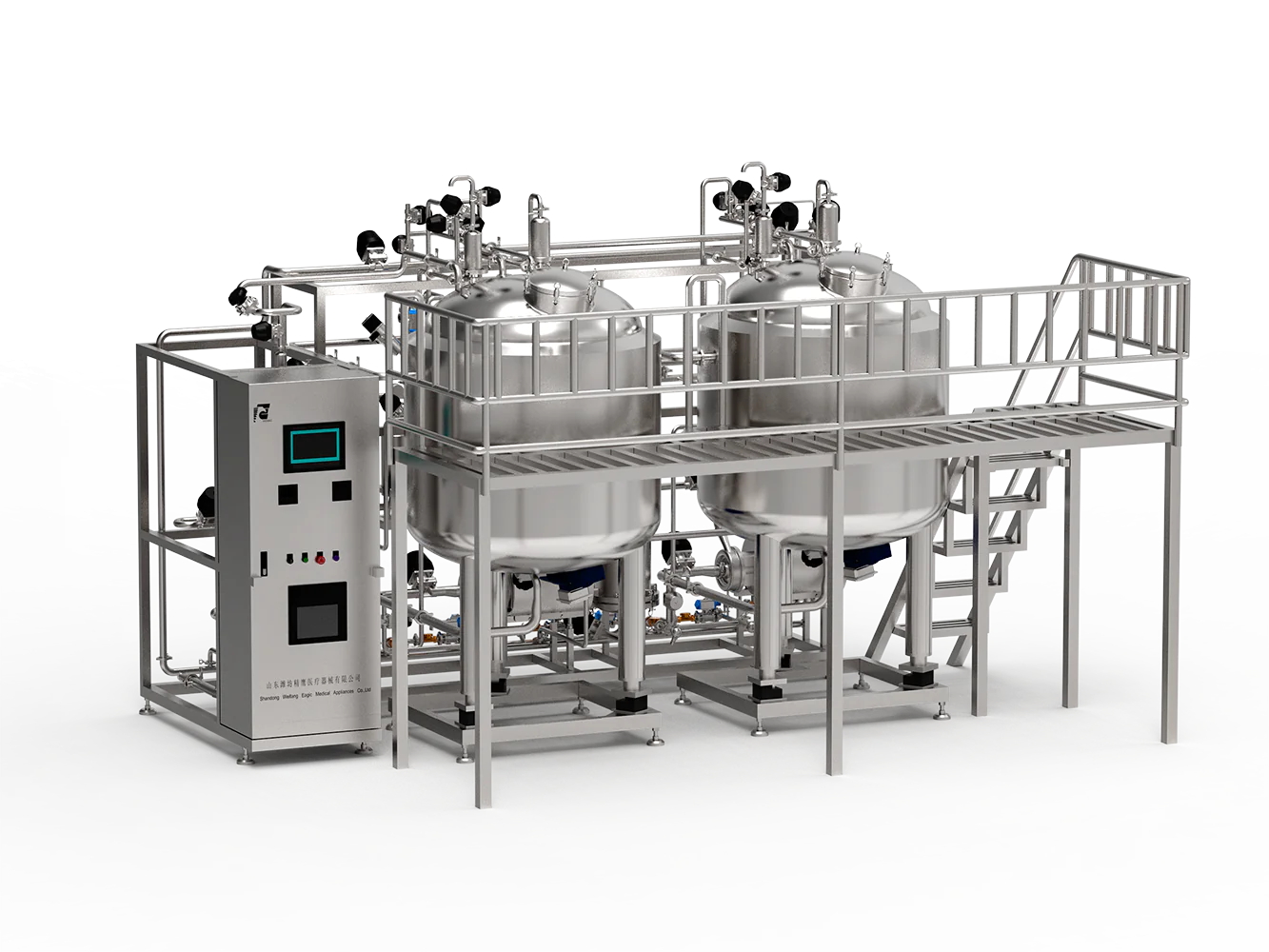
Introduction to Pharmaceutical SIP Equipment
Pharmaceutical SIP equipment, commonly referred to as Steam-In-Place sterilization systems, comprises a critical technology suite designed to ensure sterility of process piping, vessels, and ancillary systems in pharmaceutical and biopharmaceutical manufacturing environments. Their primary function is to sterilize equipment surfaces that come into contact with drug substances or intermediates without dismantling the system. This sterilization is achieved by circulating pressurized steam through sealed process assemblies to eliminate microbial contamination.
The pharmaceutical industry’s increasing emphasis on contamination control, patient safety, and product quality demands reliable sterilization systems. Pharmaceutical SIP equipment supports aseptic processing lines by enabling repeatable, validated sterilization cycles integrated within manufacturing protocols. This advanced autoclaving method contrasts with traditional steam sterilizers as it specifically targets the process equipment infrastructure and piping.
In this article, we explore the full scope of pharmaceutical SIP equipment, delving into engineering design, operational nuances, integration with CIP (Clean-In-Place) systems, regulatory compliance, and practical case studies. We will also naturally reference SKE & Eagle’s expertise in manufacturing sophisticated water and sterilization system equipment, highlighting how their engineering philosophy enhances system reliability.
Engineering Principles and Design Considerations
One critical design factor is ensuring complete steam penetration throughout the process equipment and piping, including dead legs, valves, and instrumentation ports. This is typically addressed by designing steam distribution loops with strategically placed steam injectors and condensate drains. Effective removal of air and condensate is essential since air pockets and residual moisture can significantly impair sterilization effectiveness.
Material selection is another key design consideration. Pharmaceutical SIP equipment must use corrosion-resistant stainless steels (such as 316L) and compliant gaskets compatible with steam and cleaning agents. Design for maintainability, minimal particle shedding, and cleanability aligns with Good Manufacturing Practice (GMP) requirements.
Instruments such as temperature sensors, pressure transducers, and flow meters are integrated to provide real-time process control and compliance monitoring. Advanced control systems featuring programmable logic controllers (PLCs) automate sterilization cycles while ensuring safety interlocks.
Notably, companies like SKE & Eagle incorporate robust engineering standards to develop SIP systems with superior heat transfer efficiency, system durability, and minimal footprint. Their modular skid-mounted systems exemplify industry best-practices, providing flexibility and rapid integration into existing manufacturing lines.
Operational Strategies and Validation Protocols
Successful deployment and operation of pharmaceutical SIP equipment necessitate rigorous operational strategies backed by documented validation protocols. Operation protocols define cycle parameters such as sterilization temperature, exposure time, steam quality, and pressure setpoints.
Steam quality evaluation is paramount, as superheated steam or saturated steam at proper dryness fractions is critical to sterilization efficacy. Automated purge steps precede sterilization to expel air and condensate, often verified through temperature mapping or chemical/bioindicator placement.
Validation protocols, conforming to regulatory guidelines such as USP <1211> and FDA cGMP, mandate installation qualification (IQ), operational qualification (OQ), and performance qualification (PQ). These include executing challenge tests, biological indicators assessment, and physical monitoring to confirm reproducibility and reliability.
SKE & Eagle’s comprehensive quality management system supports documentation and traceability essential for validation phases. Their control systems maintain detailed electronic batch records with secure data logging, aligning with 21 CFR Part 11 compliance requirements.
Routine maintenance and periodic re-validation also form part of the operational lifecycle, ensuring performance consistency over time and preventing bioburden resurgence. Automated diagnostic features and remote monitoring solutions improve uptime through predictive maintenance insights.
Integration with Clean-In-Place Systems
Pharmaceutical SIP equipment often operates in conjunction with Clean-In-Place (CIP) systems, which automate hygiene processes for equipment surfaces. While SIP sterilizes by steam heat, CIP uses targeted cleaning agents, detergents, and rinses to remove residues and microbial biofilms.
Seamless integration of SIP with CIP platforms optimizes plant efficiency and reduces turnaround times between production batches. Systems are engineered to switch safely and rapidly between cleaning and sterilization modes without manual disassembly, preserving aseptic conditions and minimizing downtime.
CIP-SIP integrated skid designs from providers such as SKE & Eagle include common piping manifolds, automated valving, and unified control interfaces that orchestrate complex sequences with precision. These integrated systems benefit from SKE & Eagle’s engineering culture emphasizing modularity and interoperability.
Additionally, process analytical technology (PAT) tools embedded within integrated systems enable continuous validation of cleaning and sterilization efficacy by monitoring critical parameters such as conductivity, turbidity, and steam integrity.
This integration enhances compliance with hygiene standards outlined in ISPE Good Practice Guides and Pharmaceutical Inspection Co-operation Scheme (PIC/S) expectations.
Compliance and Industry Standards
Pharmaceutical SIP equipment design and operation must comply with regulatory frameworks covering sterility assurance, equipment qualification, and safety. Globally recognized standards influencing SIP system requirements include:
- FDA Current Good Manufacturing Practice (cGMP): Mandate process validation and sterility assurance through validated sterilization cycles.
- European Medicines Agency (EMA) Guidelines: Align sterilization processes with stringent European pharmaceutical manufacturing criteria.
- International Society for Pharmaceutical Engineering (ISPE): Provides Good Practice Guides outlining best practices for sterilization technology.
- USP <1211> Sterilization and Sterility Assurance of Compendial Articles: Defines validation concepts and testing protocols.
- ASME BPE (Bioprocessing Equipment Standard): Specifies design and fabrication parameters for hygienic equipment including SIP components.
Conformance to these standards ensures product safety and regulatory approval pathways. Engineering suppliers like SKE & Eagle embed compliance considerations from the conceptual phase, incorporating documentation, material traceability, and electronics qualified per regulatory criteria.
Proper adherence to compliance also facilitates audit readiness and minimizes risk during inspections by health authorities globally.
Selecting High-Quality Pharmaceutical SIP Equipment
Choosing the right pharmaceutical SIP equipment is critical to safeguarding aseptic process integrity and manufacturing productivity. The selection process should focus on:
- System Reliability: Look for engineering excellence in materials, automation controls, and lifecycle support. Equipment from proven manufacturers like SKE & Eagle delivers superior uptime and consistent performance.
- Design Customization: Tailor SIP systems to specific process configurations and scale, avoiding one-size-fits-all solutions.
- Compliance Assurance: Validate manufacturer’s compliance with GMP and other pharmaceutical regulatory requirements.
- Integration Capability: Confirm ease of integration with existing CIP-SIP systems or other processing equipment.
- Service and Support: Ensure vendor provides comprehensive documentation, training, preventative maintenance, and rapid technical assistance.
For example, SKE & Eagle develops bespoke and modular SIP skid systems equipped with advanced instrumentation and validated controls for diverse pharmaceutical applications. Their strong focus on quality, precision engineering, and after-sales support positions them as a trusted partner.
Selecting high-quality SIP equipment ultimately mitigates contamination risk, regulatory non-compliance, and costly downtime, making it a critical investment in pharmaceutical manufacturing.
For process engineers further interested in water purification interfaces with SIP systems, explore SKE & Eagle’s Purified Water Systems product line to understand integration synergies essential to aseptic manufacturing.
Future Developments in SIP Technology
Pharmaceutical SIP equipment continues to evolve driven by innovation in automation, material science, and digitalization. Key future trends include:
- Digital Twin and Simulation Modeling: Real-time process simulation allows predictive sterilization optimization and quicker protocol development.
- Advanced Sensors and PAT Integration: Enhanced steam quality sensors and inline microbial detection methods improve process control and validation assurances.
- Energy and Water Efficiency Improvements: New cycle algorithms reduce steam consumption and condensate discharge, achieving sustainability goals.
- Smart Maintenance Programs: AI-driven maintenance forecasting reduces unplanned downtime and extends equipment life.
- Modular and Flexible SIP Systems: Adaptable platforms support multi-product manufacturing and rapid changes in process scale.
Industry leaders like SKE & Eagle are actively investing in research and development to incorporate these advances while maintaining compliance with stringent pharmaceutical quality standards. Their continuous innovation ensures customers receive future-proof sterilization solutions that blend reliability with cutting-edge technology.
Staying abreast of these developments and iterating SIP system design will enhance aseptic processing robustness and regulatory resilience for pharmaceutical manufacturers worldwide.
Frequently Asked Questions about Pharmaceutical SIP Equipment
What is the typical temperature and duration for a pharmaceutical SIP cycle?
Typically, SIP cycles use saturated steam at temperatures above 121°C (250°F) for exposure times ranging from 15 to 60 minutes, depending on equipment complexity and validation protocols to achieve sterility assurance.
How does pharmaceutical SIP equipment differ from traditional autoclaves?
Unlike traditional autoclaves that sterilize batches of instruments or containers, pharmaceutical SIP equipment sterilizes installed process piping and vessels in situ without disassembly, enabling continuous sterile processing with minimal downtime.
Can SIP systems be integrated with Clean-In-Place (CIP) processes?
Yes, many pharmaceutical manufacturers integrate SIP with CIP systems to automate sequential cleaning and sterilization, enhancing process efficiency and reducing contamination risks.
What regulatory standards apply to pharmaceutical SIP equipment?
Pharmaceutical SIP equipment design, validation, and operation comply with FDA cGMP, EMA guidelines, USP <1211>, ISPE Good Practice Guides, and ASME BPE standards to ensure sterility assurance and process control.
How does SKE & Eagle contribute to pharmaceutical SIP system excellence?
SKE & Eagle leverages advanced engineering, precision manufacturing, and rigorous quality control to supply customized SIP skid systems. Their focus on reliability, compliance, and customer support positions them as a trusted partner for pharmaceutical sterilization needs.
Contact SKE & Eagle for Expert Pharmaceutical SIP Equipment Solutions
If you seek to optimize your pharmaceutical sterilization processes with state-of-the-art SIP equipment engineered for reliability and regulatory compliance, SKE & Eagle is here to assist. Our team is ready to discuss your unique manufacturing needs and provide tailored technical expertise.
Connect with us on Facebook or email us directly at info@ske-eagle.com. We encourage you to visit our website and fill out the contact form at the bottom of the page for personalized consultation.
SKE & Eagle – Engineering excellence fueling safe and efficient pharmaceutical manufacturing.